In a previous post, I described a simplified model of Cognitive Load Theory. The advantage of the model I presented is that it allows teachers a ready framework from which to make decisions in and before class about how best to optimise their instruction for learning. This post will make more sense if you read that one first, as it concludes with the scheme below:
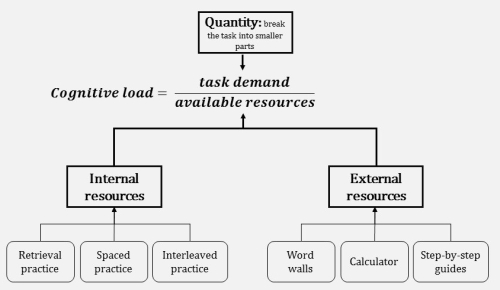
The image above represents the equation in a very general sense. The cognitive load in practicals is always going to be high. This is why general calls for your students to be “minds on” are not helpful without being provided with a specific mental model for conceptualising the load or techniques for actually minimising it. As such, I would like to propose both a mental model for the load in practicals, as well as some straightforward techniques for reducing it.
Applying our mental model to science practicals looks a bit like this:
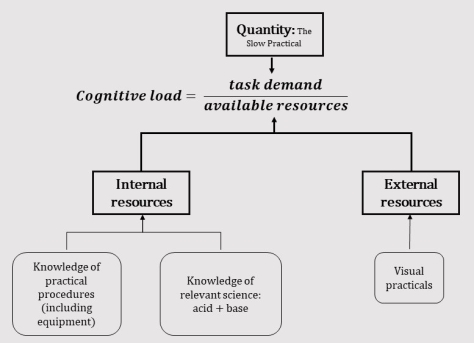
Some simple conclusions would be:
- If students lack knowledge of the relevant science during the practical, little can be learned as the available resources are low. This is why open enquiry and discovery practicals rarely work. This is why I always do practicals at the end of a scheme of work. Once I have taught the content to my students through explanation, practice, review and demonstration I do the practical: the point where their internal resources are largest.
- In general, students do not have as detailed knowledge about lab equipment as they might do about other things. It is rarely taught explicitly or re-visited as a matter of course.
- Visual practicals are a way of providing students with an external resource to lower the load. They are a part of the picture, but should not be thought of as a silver bullet due to the existence of the other variables.
Quantity is an interesting one, and generally refers to the number of things involved in a particular task. Normally, to anticipate it when teaching science content I break the content up into small segments, explain the first segment, allow students to practise it, then review. When I am happy, we move on to the next segment and repeat the process (you can read a bit more about that here).
It’s a little harder to do that with a practical, so I do something called a Slow Practical. It can be applied to any practical, but here I will use making copper sulphate as an example.
First, I gather the entire class around one table in the lab, which I call my demo table. At this table, I will demonstrate every step of the practical for the students, but in segments. In this practical, I would start with the requirement to pour a given volume of sulphuric acid. I ask students to go collect a measuring cylinder, bottle of acid, and small beaker and leave it by their place. They will quickly go and do this and return to my desk.
Back at the desk, I will show them how to decant the acid into a beaker, and then measure out the given volume. I would take the opportunity to talk to them about safety, why we use a measuring cylinder (and not say a beaker) and why it is normally best to decant the acid first (it is a lot easier to pour from a beaker than the stock bottles that we have but depends on your equipment). Students would then return to their benches, measure out their acid and then return.
I’ll repeat the process for each subsequent step of the practicals. A bit later on, I might show them how to set up their Bunsen burner and have them return to me before they are lit. I can then look over the students’ heads, have a quick glance around the empty benches (no children milling around) and check that everything is set up correctly. Throughout, I might take opportunities to discuss why it is being heated, what we are expecting to see, pick up on misconceptions like the copper oxide dissolving and to revisit material that I have taught students already about acid/base reactions, separating mixtures, excesses, colour changes, definitions of solutions etc. etc. It’s that simple. Demo, go, return. Demo, go, return. Rinse and repeat.
Advantages:
- Because you have manipulated the quantity, you have reduced cognitive load
- Students can actually learn something
- You get to ask the probing questions at a point where they aren’t crazy busy and fussing about their experiment
- You don’t end up saying the same thing to 15 different groups. You say it once, and that’s it
- You can talk to students when they are away from the fidgety distraction of their equipment
- It’s a hell of a lot calmer as students are going in one direction at once. You don’t have some students going here, some going there and generally causing chaos
- Because students know exactly what they are supposed to be doing, it’s a lot more purposeful. Students don’t end up milling around or doing nothing and chatting to their friends while they are waiting for you to come over and help
- Chance for error is a lot smaller. Because they have just seen you do it, and can always just look back to the demo table, they are more likely to actually carry out the procedure correctly than following paper instructions (even when visual)
- Consequently, it’s also a lot safer
Before I did slow practicals I was always exhausted after a practical. Constantly running round, correcting equipment, calling across a legitimately busy classroom to students who were being illegitimately un-busy, repeating the same thing a million times and just generally lots of craziness, all followed by having to clear up a ton of mess. To boot, nobody learnt anything, and students had nary a clue why they were doing what they were doing.
Now, practicals are a walk in the park. I rarely even leave the demo table: I can see really quickly and easily who is doing it right and who is not, and it’s significantly easier to communicate to individual students or the entire group. My students carry out methods with greater skill and are pushed to actually think about what they are doing – all because I have manipulated the variables in my cognitive load equation.
The disadvantage of course is that it is slower. It takes a bit more time (though not always), and practical lessons can be tight. But then they were pretty tight before, and I would argue that if students aren’t actually learning from the practical, what is the virtue of it fitting neatly into a 55 minute slot?
December 6, 2018 at 10:16 pm
Really nice. In my thesis in 1991, I referred and made use of something similar namely the experimental seminar as first discussed by Conway, Mendoza, and Read (1963). Keep up the great work.
LikeLiked by 2 people
December 6, 2018 at 10:17 pm
Thanks Paul! I’ll check that out 🙂
LikeLike
December 7, 2018 at 3:27 pm
Really interesting, thank you. I’m looking at using dataloggers to reduce cognitive load in science practicals for my MA dissertation. I contacted Sweller for his thoughts and he suggested I look at the Redundancy effect too. Made for interesting reading.
LikeLike
December 8, 2018 at 4:56 pm
Nice! Will be interested to see what you come back with
LikeLike
December 7, 2018 at 4:20 pm
Very logical and rings true. I have arrived by trial and error over a long career at this conclusion also, great to see others thinking deeply aBout the same issues. Thank you
LikeLike
January 4, 2019 at 12:24 pm
Reblogged this on The Echo Chamber.
LikeLike
July 12, 2019 at 9:11 am
Hi Adam, I like the idea pedagogical, my only concern would be that if you demo it in chunks, after every chunk, all students have to wait for the slowest student. Do you have any suggestions for mitigating this?
LikeLiked by 1 person
July 12, 2019 at 9:50 am
What I’ve found is that they all end up working at roughly the same speed – if they aren’t you can either:
1) increase the speed of the slowest. I find that a well aimed b*llocking can help here.
2) respond to the fastest, I will sometimes have them come back to the main desk and then just hit them with some verbal retrieval Qs, or have a bank ready for them to do in their books inbetween steps.
Neither ideal, but overall the downsides are FAR outweighed by the upsides!
LikeLike
January 2, 2020 at 11:36 am
Yes I agree. This is something design and technology/ food teaching has always done otherwise there would be very poor outcomes. Ask a practical teacher learning through application= technology
LikeLiked by 1 person
September 1, 2021 at 1:19 pm
This makes a lot of sense; thank you. I will definitely discuss this with my Science Department, while we use the Ofsted Research Review for Science to evaluate our practice. Thank you very much!
LikeLiked by 1 person